Blog
Understanding PP Yellowing: Causes, Prevention, and Solutions for Polypropylene
Polypropylene (PP) is one of the most widely used thermoplastic polymers globally, valued for its low cost, high heat resistance, and good mechanical properties. However, a common and critical issue faced by manufacturers and end-users alike is “PP yellowing,” the undesirable discoloration of the material towards a yellow or brownish tint. This aesthetic defect often indicates underlying chemical degradation and can compromise both the appearance and physical integrity of the final product.
Understanding the causes of this phenomenon and implementing appropriate preventative measures and solutions is essential for maintaining product quality and longevity.
What is PP Yellowing?

PP yellowing is a visual indicator of polymer degradation. The process involves chemical changes within the polypropylene’s molecular structure under the influence of external factors like heat, oxygen, and UV light
Polypropylene, in its pure, natural state (without additives), is inherently unstable and highly susceptible to oxidation. The polymer chains break down, forming new molecular arrangements called “chromophores,” which absorb blue and violet light and reflect yellow light, thus causing the material to appear yellow. This process is irreversible if the polymer structure itself is altered, though surface stains or additive migration can sometimes be cleaned or addressed.
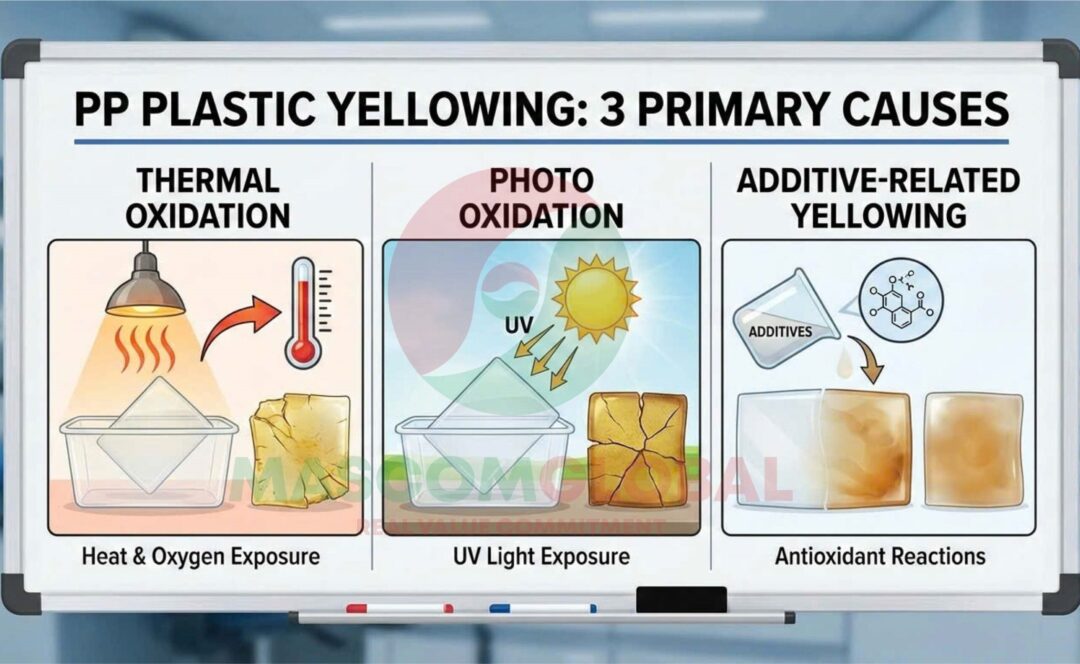
Primary Causes of PP Yellowing
PP yellowing generally stems from three main mechanisms of degradation: thermal oxidation, photo-oxidation (UV exposure), and chemical interactions, often involving additives themselves.
-
Thermal Oxidation (Heat and Oxygen)
Heat is a primary driver of degradation, particularly during processing (extrusion, injection molding) where high temperatures are necessary. When combined with oxygen in the air, the heat causes a process called thermal oxidation. This reaction creates free radicals, which initiate a chain reaction that breaks down the polymer chains, ultimately forming the yellowing chromophores. Overheating or excessive shear during processing can accelerate this effect.
-
Photo-Oxidation (UV Light Exposure)
Polypropylene is sensitive to UV light. The high energy from UV radiation can break the chemical bonds within the PP polymer chains, generating free radicals. These radicals then react with oxygen to form hydroperoxides and carbonyl groups, leading to oxidation and subsequent yellowing. Continuous exposure to sunlight or certain artificial light sources is a significant factor in products intended for outdoor use.
-
Additive-Related Yellowing (Gas Fading and Side Effects)
Ironically, some additives designed to protect the plastic can, under certain conditions, contribute to yellowing.
- Phenolic Antioxidants: These are common stabilizers used to inhibit degradation during high-temperature processing. However, under long-term exposure to certain atmospheric pollutants, such as nitrogen oxides (NOx) found in industrial areas or from forklift traffic, these antioxidants can over-oxidize and form quinone structures, which are inherently yellow or brown. This is a specific phenomenon often referred to as “gas fading.”
- Migration (Blooming): Sometimes, additives or their degradation byproducts can migrate to the surface of the plastic product, creating a visible yellowish stain that cannot be wiped off.
Solutions and Prevention Strategies
Preventing PP yellowing requires a proactive approach that addresses the causes during material selection, manufacturing, and storage.
-
Material Selection and Stabilization
The most effective way to combat yellowing is by using the right stabilization package from the start.
- Antioxidant Systems: Incorporating a carefully balanced blend of primary (phenolic) and secondary antioxidants is crucial for thermal stability during processing and long-term use. Suppliers like Mascom Global provide specialized compounding solutions with optimized additive packages.
- UV Stabilizers (HALS): For applications exposed to sunlight, specific UV inhibitors or Hindered Amine Light Stabilizers (HALS) must be added during compounding to prevent photo-oxidation.
- Anti-Gas Fading Agents: Using specific stabilizers, sometimes zinc-based compounds, can neutralize the colored byproducts of phenolic antioxidants reacting with NOx gasses.
-
Optimized Processing Conditions
Controlling the manufacturing environment helps minimize thermal degradation:
- Temperature Control: Ensure processing temperatures are optimized and do not exceed the recommended limits for the specific grade of PP.
- Minimize Residence Time: Reduce the amount of time the molten plastic sits in the machine barrel to avoid prolonged heat exposure.
- Reduce Oxygen Exposure: In some specialized drying or molding processes, replacing oxygen with nitrogen can significantly reduce thermal oxidation.
-
Proper Storage and Handling
Post-production storage conditions play a vital role in preventing premature yellowing:
- Store Away from Light and Heat: Keep PP products in a cool, dry, and dark place to minimize exposure to UV light, visible light, and high temperatures.
- Control Atmosphere: If possible, avoid storage areas with high concentrations of industrial pollutants (e.g., near running forklifts or gas heaters) to prevent gas fading.
By addressing these factors, manufacturers can ensure their polypropylene products maintain their intended color and performance properties throughout their intended lifespan. Mascom Global is dedicated to providing high-quality masterbatches and functional compounds that address the challenges of PP yellowing, ensuring durable and aesthetically pleasing results for our global customers.

